
A study in ScienceDirect explains that methanotrophic bacteria has the ability to ‘utilize methane as their sole carbon and energy source.’ And what this partially means is that it can consume at least 30 million metric tons of methane per year, which is something that has interested scientists and researchers due to its natural ability to convert this potent greenhouse gas into usable fuel.
Yet regardless of this amazing ability, scientists still don’t know much about how such a complex reaction actually occurs, which in turn limits the ability of researchers to use this benefit to the best of the planet’s advantage.
A team at Northwestern University has been studying the enzyme that the bacteria uses to catalyze the reaction, and has since discovered the key structures that may possibly be driving this process in the first place.
What these findings mean is that they could eventually ‘lead to the development of human-made biological catalysts that convert methane gas into methanol.’
Senior author of the paper, Amy Rosenzweig, from Northwestern, explained, “Methane has a very strong bond, so it’s pretty remarkable there’s an enzyme that can do this. If we don’t understand exactly how the enzyme performs this difficult chemistry, we’re not going to be able to engineer and optimize it for biotechnological applications.”
The enzyme in question is called particulate methane monooxygenase (pMMO), which is a rather difficult protein to study since it’s ‘embedded in the cell membrane of the bacteria.’
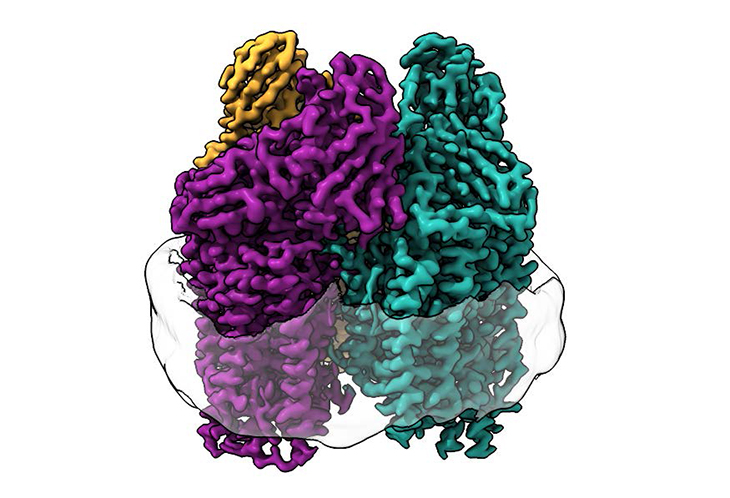
Normally when the scientists study the methanotrophic bacteria, they use a harsh process where they use a detergent solution to rip out of the cell membranes. Although the procedure manages to isolate the enzyme effectively, it tends to kill the enzyme activity, which then limits the amount of information that the researchers can gather. They liken it to monitoring a heart without the heart having a heartbeat.
For this particular study, the team used a different and new technique completely. The first author, Christopher Koo, who happens to be a Ph.D. candidate in Rosenzweig’s lab, thought about what would happen if they put the enzyme back into a membrane that also resembles the native environment, to see if they could possible learn something new. Rather, Koo decided to use lipids from the bacteria to form a membrane within the protective particle called a nanodisc, after which, he embedded the enzyme into the membrane.
Koo shared, “By recreating the enzyme’s native environment within the nanodisc, we were able to restore activity to the enzyme. Then, we were able to use structural techniques to determine at the atomic level how the lipid bilayer restored activity. In doing so, we discovered the full arrangement of the copper site in the enzyme where methane oxidation likely occurs.”
The researchers also share that they use a particular type of technique called cryo-electron microscopy (cryo-EM), which is known to be well-suited to membrane proteins ‘because the lipid membrane environment is undisturbed throughout the experiment.’ What this did was allow them to ‘visualize the atomic structure of the active enzyme at high resolution for the first time.’
Rosenzweig said, “As a consequence of the recent ‘resolution revolution’ in cryo-EM, we were able to see the structure in atomic detail. What we saw completely changed the way we were thinking about the active site of this enzyme.”
Rosenzweig explained that the cryo-EM structures give a new starting point to provide answers to the questions that are still coming forward, such as ‘How does methane travel to the enzyme active site? Or methanol travel out of the enzyme? How does the copper in the active site do the chemical reaction? Next, the team plans to study the enzyme directly within the bacterial cell using a forefront imaging technique called cryo-electron tomography (cryo-ET).’
Should the study be successful, the research group will be able to see just how the enzyme is arranged in the cell membrane, as well as figure out how it will operate in its true and native environment. They will also figure out if the other proteins that surround the enzyme actually interact with it or not. All of these discoveries will also provide vital notes to engineers.
Huge Potential to Clean Up Oil Spills
“If you want to optimize the enzyme to plug it into biomanufacturing pathways or to consume pollutants other than methane, then we need to know what it looks like in its native environment and where the methane binds. You could use bacteria with an engineered enzyme to harvest methane from fracking sites or to clean up oil spills,” added Rosenzweig.
You may read more about the research study in the journal, Science.
What are your thoughts? Please comment below and share this news!
True Activist / Report a typo


