A research group from the U.S. Department of Energy’s SLAC National Accelerator Laboratory and Stanford University have joined together to find a way to revive rechargeable lithium batteries. What this could mean is to possibly boost the range of battery life for the next generation of electric vehicles and electronic devices.
When it comes to the lifecycle of lithium batteries, they eventually accumulate little islands of inactive lithium that end up being cut off from the electrodes, which eventually decreases the capacity of the battery’s overall store charge.
What the research group figured out is a way to make these “dead” lithium slowly move towards the other electrodes, much like a worm inching it’s way over, until it manages to reconnect, allowing it to partially reserve the parts that have, in essence, “died.”
By doing this, they managed to slow down the degradation of the test battery, which managed to increase the lifetime by almost 30%.
Published in Nature journal last December 22, the lead author of the study, Stanford postdoctoral fellow Fang Liu, said, “We are now exploring the potential recovery of lost capacity in lithium-ion batteries using an extremely fast discharging step.”
There is a lot of ongoing research that’s looking for new and innovative ways to develop rechargeable batteries with certain characteristics like longer lifetimes, lighter weight, faster charging speeds than the lithium-ion technology used in smartphones, laptops, and electric vehicles, and improved safety as well.
One main focus has been on developing lithium-metal batteries that could store more energy per volume or weight. One example of this would be in electric cars, where these so-called next-gen batteries could possibly increase the EV’s mileage per charge, while also taking up less trunk space.
For both lithium-ion and lithium-metal batteries, they use positively charged lithium ions that travel back and forth between the electrodes. As time goes on, some of the metallic lithium becomes electrochemically inactive, which end up forming ‘isolated islands of lithium’ that don’t connect with the electrodes. As a result, there is also a loss of capacity of the battery, which tends to be the usual problem when it comes to lithium-metal technology, as well as for all the other fast charging lithium-ion batteries on the market.
But in this new study, the research group showed how they managed to mobilize and recover these isolated lithium islands in order to extend the battery life.
Professor at Stanford and SLAC and investigator with the Stanford Institute for Materials and Energy Research (SIMES), Yi Cui, who happened to lead the research, shared, “I always thought of isolated lithium as bad, since it causes batteries to decay and even catch on fire. But we have discovered how to electrically reconnect this ‘dead’ lithium with the negative electrode to reactivate it.”
Not Completely Dead
As explained by Prof. Cui, he believes that ‘applying a voltage to a battery’s cathode and anode could make an isolated island of lithium physically move between the electrodes – a process his team has now confirmed with their experiments.’
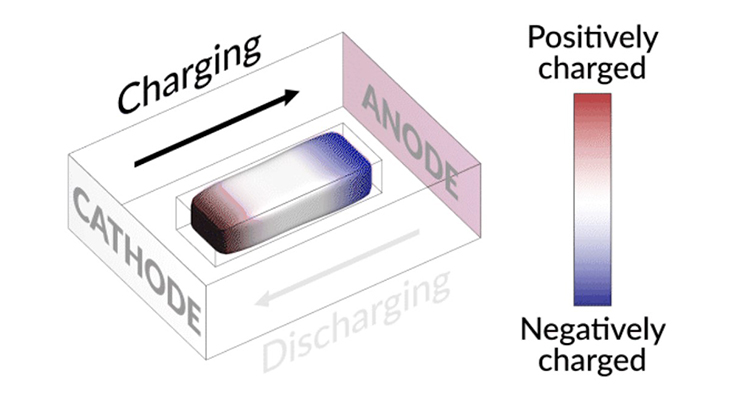
The scientists invented an optical cell with a lithium-nickel-manganese-cobalt-oxide (NMC) cathode, a lithium anode, and an isolated lithium island in between. This fabricated test device let them track what happens inside a battery when it’s being used in real time.
What they found was that the isolated lithium island wasn’t actually “dead,” but still responded to the operations of the battery. When they charged the cell, the island was seen slowly moving towards the cathode, while during discharge, it would creep in the opposite direction.
Prof. Cui explained, “It’s like a very slow worm that inches its head forward and pulls its tail in to move nanometer by nanometer. In this case, it transport by dissolving away on end and depositing material to the other end. If we can keep the lithium worm moving, it will eventually touch the anode and reestablish the electrical connection.”
After getting the results, the research group managed to validate the outcome with other test batteries, as well as through computer simulations, to demonstrate how the isolated lithium could be recovered in a real battery just by changing the charging protocols of the battery.
Liu chimed in, sharing, “We found that we can move the detached lithium toward the anode during discharging, and these motions are faster under higher currents. So we added a fast, high-current discharging step right after the battery charges, which moved the isolated lithium far enough to reconnect it with the anode. This reactivates the lithium so it can participate in the life of the battery.”
In conclusion, Liu said, “Our findings also have wide implications for the design and development of more robust lithium-metal batteries.”
What are your thoughts? Please comment below and share this news!
True Activist / Report a typo